Common gases and their applications
oxygen: breathing / respiration acetylene: welding carbon dioxide: photosynthesis / combustion argon: incandescent light bulbs neon: signs chlorine: water treatment nitrogen: Haber process (making ammonia) helium: birthday balloons hydrogen: fuel cells methane: natural gas ozone: UV shield for Earth propane: barbecue fuel
The Earth’s Atmosphere
N : 78.1% of Earth’s Atmosphere O : 20.9% of Earth’s Atmosphere Ar : 0.93% of Earth’s Atmosphere CO : 0.03% of Earth’s Atmosphere Ne: 0.002% of Earth’s Atmosphere He: 0.0005% of Earth’s Atmosphere
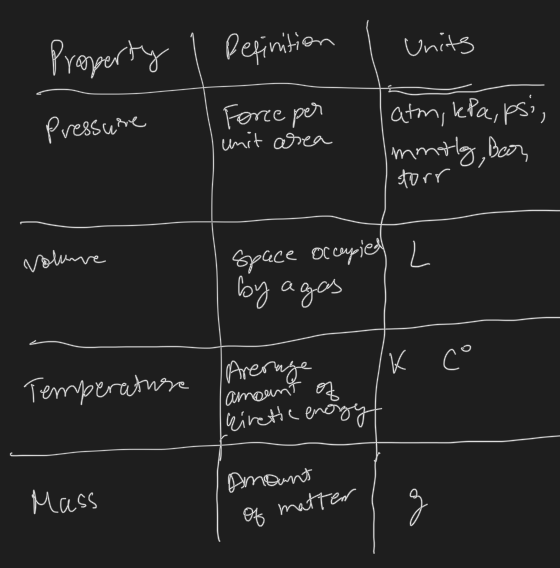
List the macroscopic properties of gases
- temperate
- color
- melting point
- boiling point
- mass
- shape
- density
- volume
- pressure
- compressibility
Quantitative Properties of Gases
Kinetic Molecular Theory
- The volume of individual gas molecules is negligible compared to the volume of the container holding the gas. Gas molecule are, therefore, extremely far apart and most of the container is empty space
- There are neither attractive nor repulsive forces between gas molecules
- Gas molecules have high translational energy (means move really really fast). They move randomly in all directions and in straight lines
- When gas molecules collide with each other or with the container walls, the collisions are entirely elastic. There is no loss of kinetic energy (don’t lose energy or gain energy).
- The average kinetic energy of gas molecules is directly related to the temperature of the gas
Types of Motion in gases:
Vibrational - molecules vibrate Rotational - molecules rotate in their positions & are able to change position Translational - molecules move on their own in different direction
Pressure
Pressure is measured in various units mmHg (millimeters of mercury) torr (named after italian physicist Evangelista Torricelli) atm (atomospheres) psi (pounds per square inch) Pa (pascals) or kPa (kilopascals)
1 atm = 760 mmHg = 760 torr = 14.7 psi = 101.3 kPa = 1.013 Bars
Boyle’s Law
In 1662 Robert Boyle stated that the volume of a given amount of gas, at a constant temperature, varies inversely with the applied pressure
V propotional 1/P OR PV = k
so… P V = P V
Example: A balloon with a volume of 5.0 L is filled with air at 101 kPa. The balloon is taken up to the mountains where the atomospheric pressure is 682.7 mmHg. If the temperature stays the same in both places what will the new volume be? P = 101 kPa V = 5.0 L P = 682.7 mmHg * = 91 kPa (using conversion rate 101.3 kPa = 760 mmHg)
P V = P V
V = = 5.5 L
STP - Standard Temperature Pressure
O C and 1 atm
SATP - Standard Ambient Temperature Pressure
25 C and 100 kPa
Homework (Grade 11 Chemistry)
P.514 # 1 - 10 P.515 # 1 - 14
Jacques Charles and Lord Kelvin
- as gases are cooled their volumes decrease
- for each degree Celsius the temperature is decreased, the volume decreases by a factor of 1/273
- Lord Kelvin realized that -273 C, molecular motion would theoretically cease
- He developed the Kelvin temperature Cale
- T = C + 273
- Today we used the accepted relationship of
- T = C + 273.15
Charle’s Law (volume and temperature)
The volume of a fixed mass of gas at constant pressure is directly proportional to its Kelvin temperature
= k so we get =
… So we need to use the Kelvin temperature scale for all these calculations. (Some conversions may be necessary)
A balloon is filled with helium gas to a volume of 1.20 L at a pressure of 105 kPa and a temperature of 15 C. If the pressure remains constant and the temperature rises to 303 K, what will be the new volume of the balloon?
T = 15C + 273 K = 288 K T = 303 K V = 1.20 L
= 1.26 L
Grade 11 Chemistry
- P.522 # 11 - 20
Gay-Lussac’s Law (pressure and temperature)
The pressure of a fixed amount of gas, at constant volume, is directly proportional to its Kelvin temperature
P = KT => P/T = K
=
all temperatures must be convered to Kelvin because cannot divide by 0 and it makes more sense to use Kelvin
A gas cylinder is stored in a room that is concrete lined for safety. The cylinder is designed to withstand 50 atm of pressure. The pressure gauge reads 35 atm at 23.2 C. A fire in the next room raises the temperature in this room to 87.5 C. What will the pressure gauge read at this temperature
Converting from Celsius -> Kelvin add 273 Converting from Kelvin -> Celsius subtract 273
T = 23.2 C + 273 = 296.2 K P = 35 atm T = 87.5 C + 273 = 360.5 K P = ?
P = = = 42.6 atm = 43 atm
Homework: p.525 # 21 - 30 p.527 # 1 - 14
Combined Gas Law
If we combine Boyle’s, Charles’, and Gay-Lussac’s Laws, we can compare pressure, volume, and temperature together
=
Homework: p.542 # 1 - 10
Law of Combining Values
When gases react, the volumes of the gaseous reactants and products, measured under the same condition of pressure and temperature, are always in whole-number ratios.
Avogadro’s Law
Equal volumes of gases contain the same number of particles, regardless of their mass
The molar volume of a gas:
- atSTP -> 22.4 L / mol
- at SATP -> 24.8 L / mol
The Ideal Gas Law
- An Ideal gas is a gas that conforms to the assumptions of the KMT and follows gas laws exactly (there is no such gas)
- Most gases will behave as ideal gases if high pressure and low temperatures are avoided
= * 1 mol
Replaces 1 mol with n moles
= * n mol
Rearrange so all symbols are on the left hand side,
= Universal Gas Constant = R
PV = nRT
R = 8.314 () = 0.08206 ()
depending on pressure unit used (kPa or atm)
Example: A 2.50 L container is filled with sulfur dioxide at a pressure of 120.0 kPa and a temperature of 27.0 C. Calculate the mass of sulfur dioxide gas in the container
Given: V = 2.50 L T = 27.0 C = 300 K P = 120.0 kPa
- Calculate n (# of moles)
- Calculate mass
n = = n = nM = (0.12027…)(64.06) = 7.7050… g = 7.71 g
Homework: p.549 # 11 - 20 p.550 # 1 - 14 p.556 # 21 - 30
Dalton’s Law of Partial Pressures
John Dalton studied the effect of gases in a mixture. He observed that the Total Pressure of a gas was the sum of the Partial Pressure of each gas
P_T$$_o$$_t$$_a$$_l = P + P + P … P
Example: A mixture of gases contain 20% He, 50% Ne, and 30% Rn. If the total pressure of the gases is 225 kPa, what is the partial pressure of each gas
He: 0.20 * 225 kPa = 45 kPa Ne: 0.50 * 225 kPa = 112.5 kPa Rn: 0.30 * 225 kPa = 67.5 kPa
Vapor Pressure
All liquids have a gas-liquid interface where there is a dynamic equilibrium between molecules in the gas phase and the liquid phase
The Boiling Point of a liquid is defined as the temperature at which the vapor pressure of the liquid is equal to the atmospheric pressure above the liquid
Gas Law Stoichiometry
Now we can use what we’ve learned about gas laws in stoichiometric calculations.
Example:
Ammonia is produced by the direct combination of hydrogen gas with nitrogen gas. If 12.0 L of nitrogen gas reacts with hydrogen gas at the same temperature and pressure, what volume of ammonia is produced and what is the volume of hydrogen used up?
Step 1: 3H + N -> 2NH
Step 2: Remember that mole ratios of the volumes of gases is the same as the ratios of the volumes. Avogadro’s Rule
Step 3: V = V * 2 = 12.0 L * 2 = 24.0 L
Step 4: V = V * 3 = 12.0 L * 3 = 36.0 L
What volume of gas is produced when excess sulfuric acid reacts with 40.0 g of iron at 18.0 C and 100.3 kPa? (The iron compound produced is an iron (II) compound)
HSO + Fe -> FeSO + H
n = m/M = 40.0 g / 55.85 = 0.716204… mol
n = n * 1/1 = 0.716… mol
V = nRT/P = 17.3 L
Homework (Grade 11 Chemistry)
P.560 # 31 - 40 p.563 # 1 - 14
Single displacement reaction lab Double displacement reaction lab Dilution Lab Certain # of moles of a compound lab