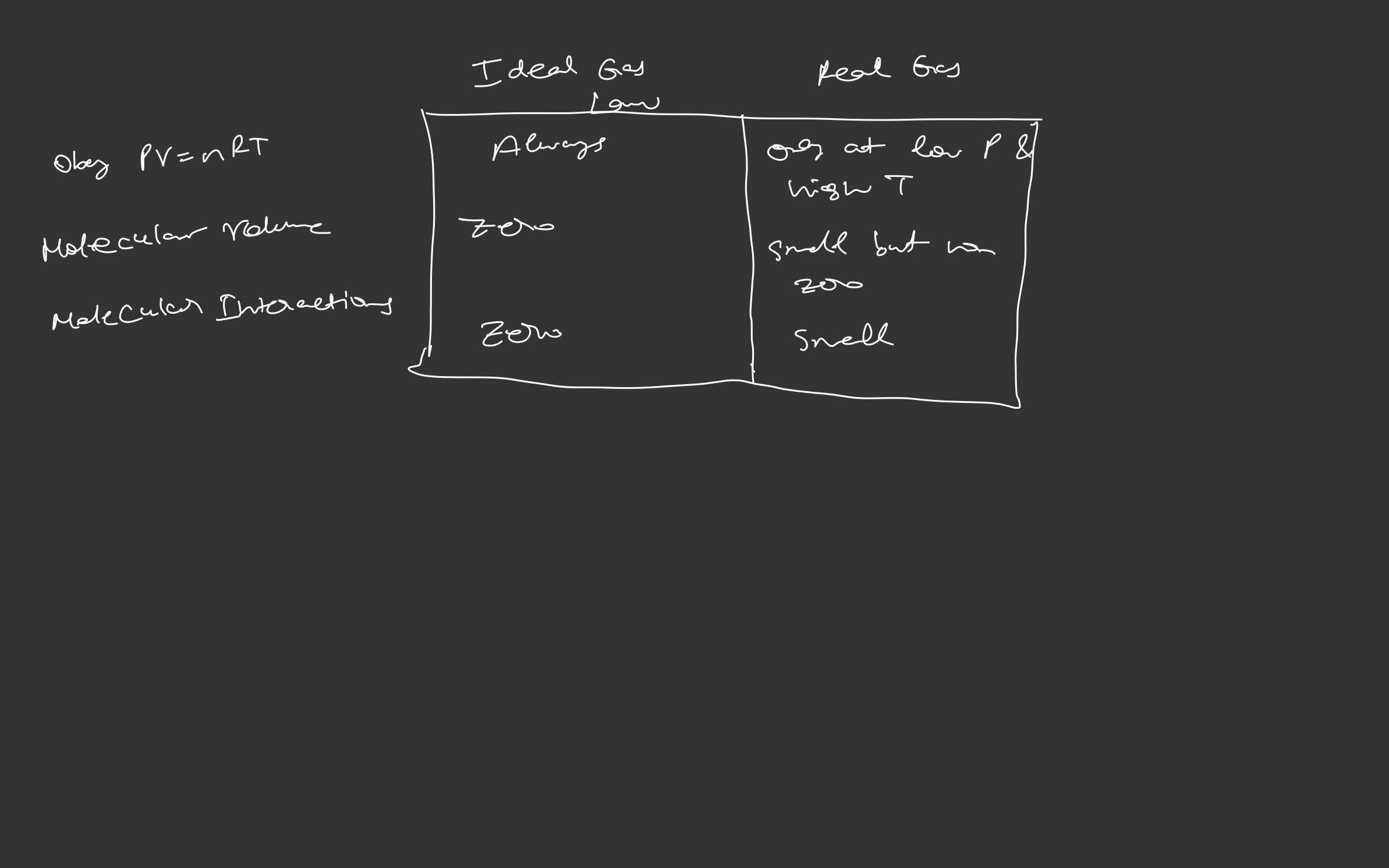
Kinetic Molecular Theory: Ideal gas laws assume
- no attraction between gas molecules
- gas molecules do not take up space
When does this happen?
- low pressures AND high temperatures
- At high pressures and low temperatures these assumptions are not valid
Ayush’s terms: low pressure and high temperatures meaning more volume and / or less of something
Volume of Gas Particles
As the container volume decreases the pressure becomes greater than of an ideal gas.
- Small container volumes -> gas particles occupy a larger % of the volume -> less space to move -> more collisions with the container -> actual pressure greater than predicted by ideal gas law
Not all gases deviate the same, 1 mole of methane would deviate more than 1 mole of helium
Larger molecules deviate most from ideal behavior
Intermolecular Attractions
If experimental value is higher = Volume issue
If experimental value is lower = Intermolecular issue
Lower temperatures -> gas particles move slower -> spend more time interacting with each other -> less time colliding with the walls -> actual pressure less than that predicted by the ideal gas law
- Polar molecules deviate most from ideal behavior
Differences
Ideal gases obey all the statements of kinetic theory. However, we know that gas particles (atoms or molecules) do have a volume of their own, and there are attractions between particles, even though gas particles are widely separated from each other.